
BOOST: a rapid ten-fold expansion method on hour-scale
We have developed an ultrafast and robust expansion microscopy workflow named BOOST, which leverages a series of novel microwave-accelerated ExM chemistry, resulting in a single-step linear expansion of tenfold on hour-scale. Specifically, BOOST facilitates a tenfold expansion of cultured cells, tissue sections, and even the challenging-to-expand FFPE sections under merely 90 minutes. Furthermore, BOOST employs microwave-assisted proteomic staining and immunostaining to facilitate high-resolution visualization of structural and molecular details with significantly enhanced throughput. Notably, BOOST has achieved a ~tenfold expansion of large millimeter-sized three-dimensional specimens in approximately three hours. BOOST offers an easily adaptable workflow based on stable and common reagents.
Ultrafast ExM workflow for diverse biological samples

Fast microwave-assisted chemistry for expansion microscopy

BOOST's improved throughput was largely due to the innovative implementation of microwave-compatible ExM chemistries. Microwave irradiation accelerates monomer infiltration, anchoring, gelation, denaturation/hydrolysis, and staining chemistries, with the denaturation step accelerating the most significantly.
BOOST Demonstrations
BOOST is compatible with post-denaturation morphological staining

3D visualization of a mitotic U2-OS cell stained with ATTO 565 NHS ester (magenta) and SYTOX Green (cyan).
Expansion factor = 10.2×
VT-iSIM microscope with a 60×/1.20 NA water immersion objective.
BOOST is compatible with post-denaturation morphological staining

3D visualization of a mouse brain section stained with ATTO 488 NHS ester (green).
Expansion factor = 9.1×
ZEISS LSM900 microscope with a 40×/1.0 NA water immersion objective.
BOOST is compatible with post-denaturation immunostaining

3D visualization of a normal U2-OS cell stained against alpha-tubulin (red).
Expansion factor = 9.1×
ZEISS LSM900 microscope with a 40×/1.0 NA water immersion objective
Large sample 10-fold expansion
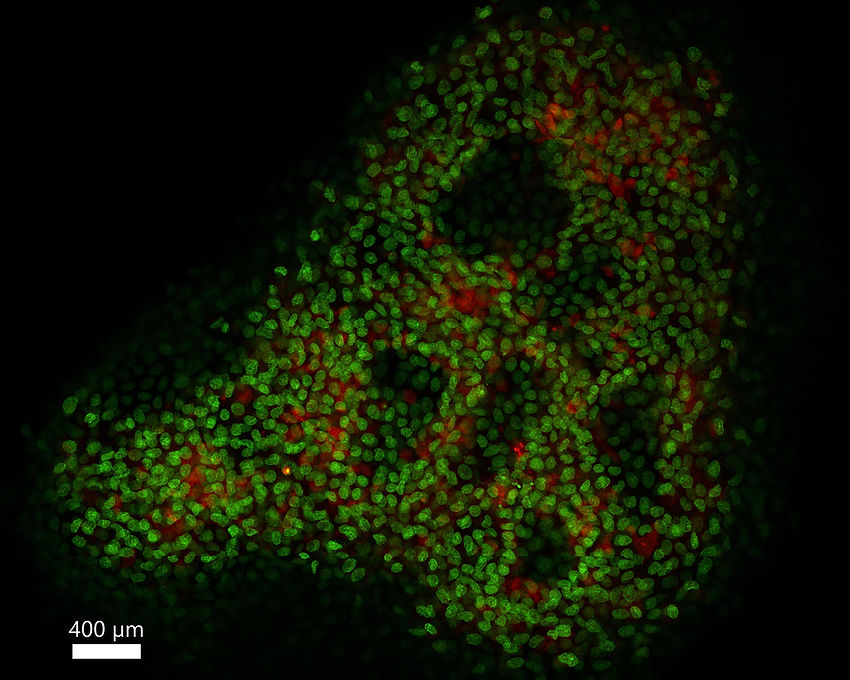
Expanded airway organoid stained with ATTO 565 NHS ester (red) and SYTOX Green green).
Expansion factor = 10.1×
ZEISS LSM900 microscope with a 2.5×/0.085 NA air objective
Large sample 10-fold expansion

Expanded airway organoid stained with ATTO 565 NHS ester (red) and SYTOX Green green).
Expansion factor = 10.1×
ZEISS LSM900 microscope with a 10×/0.3 NA water immersion objective
Firm gel without addition of sodium acrylate, expanded tenfold

Step by step workflow
BOOST stock solution prepearation

*BOOST solution can be prepared in bulk and stored at 4˚C for up to one month
Gelation chamber

option 1: sigmacoted slide applied with silicone sheet of desired thickness

option 2: parafilm wrapped slide applied with silicone sheet of desired thickness
Step 1: Anchoring






Anchoring process microwave set-up with a fixed mouse brain section as an example
Step 2: Gelation





Gelation process microwave set-up with mouse brain section as an example
Step 3: Hydrolysis/Denaturation

*Hydrolysis can be prepared and stored at room temperature for up to two months, check pH upon usage.





Hydrolysis / denaturation process microwave set-up with mouse brain section as an example
Step 4: Wash & Expand


2.5× expansion in PBS

10× expansion

10× expansion
Wash and expand process with a mouse brain section as an example
Step 5: Staining
Optional staining 1 : NHS ester + SYTOX Green morphological staining

The denatured gel after thorough washing or shrunk back in PBS after expansion, was incubated with NHS ester ATTO dyes diluted in 1:200 in PBS and/or SYTOX Green dyes diluted in 1:500 under microwave irradiation. Then, the gel was washed in large volumes of PBST for at least two times, 5-10 minutes each varying on sample size. For expansion, please refer to step 4.
Optional staining 2 : Immunofluorescence staining


The denatured gel after thorough washing or shrunk back in PBS after expansion, was incubated in the blocking buffer under microwave irradiation for 10 minutes. Then, the gel was incubated with primary antibodies diluted 1:100 normally under programmed microwave irradiation. The gel was then washed three times with excessive PBS with 0.1% Tween20 (PBST), 5 minutes each. The gel was finally incubated with fluorophore-conjugated secondary antibodies (1:150 dilution normally) under programmed microwave irradiation. The gel was then washed for at least three times with excessive PBST, 5 minutes each. For expansion, please refer to step 4.


